Mucopolysaccharidosis IIIA (MPSIIIA), also known as Sanfilippo syndrome A, is an autosomal recessive lysosomal storage disorder. The disease is characterized by a severe and progressive loss of cognitive and motor functions, behavioral deficits and eventually death in the second decade of life, although the severity and progression of the disease varies widely. MPSIIIA is caused by mutations in the SGSH gene that result in deficiency of the N-sulfoglucosamine sulfohydrolase enzyme and subsequent accumulation of undegraded heparan sulfate, lysosomal enlargement, as well as cellular and organ dysfunction.
MPSIIIA mice (JAX#003780) contain a spontaneous Sgsh mutation, resulting in an almost complete loss of N-sulfoglucosamine sulfohydrolase activity.
The most important characteristics of MPSIIIA mice are:
- Memory deficits
- Social behavior deficits
- Reduced general activity
- Increased body weight
- Neuroinflammation
- Lysosomal pathology
Evaluation of the approach in the three chamber social interaction test showed that MPSIIIA mice spent less time approaching a stranger mouse compared to wild type mice (Fig.1A). Analysis of MPSIIIA mice in the Barnes maze test showed that MPSIIIA mice spent less time in the target quadrant during the probe trial compared to wild type mice (Fig.1B). Both analyses were performed with MPSIIIA mice at the age of 30 weeks. Results show, that MPSIIIA mice present social and cognitive deficits.
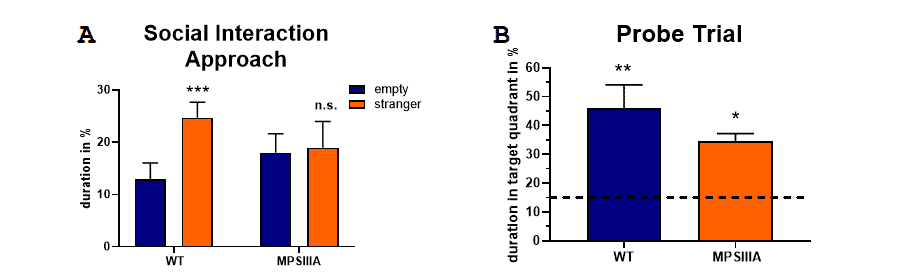
Figure 1: Behavioral analyses of MPSIIIA mice compared to wild type (WT) littermates in the three chamber social test and Barnes maze test. A: social approach in the social interaction test and (B) time animals spent in the target quadrant during the probe trial of the Barnes maze test at the age of 30 weeks. A: Two-Way ANOVA with Bonferroni’s post hoc test. B: One sample t-test; significances present differences compared to chance rate of 25% (dotted line); *p<0.05; **p<0.01; ***p<0.0001.
Histological evaluation of astrogliosis by GFAP labeling showed a highly increased GFAP-positive immunoreactive area in the cortex of MPSIIIA mice compared to wild type mice (Fig.2A and B).
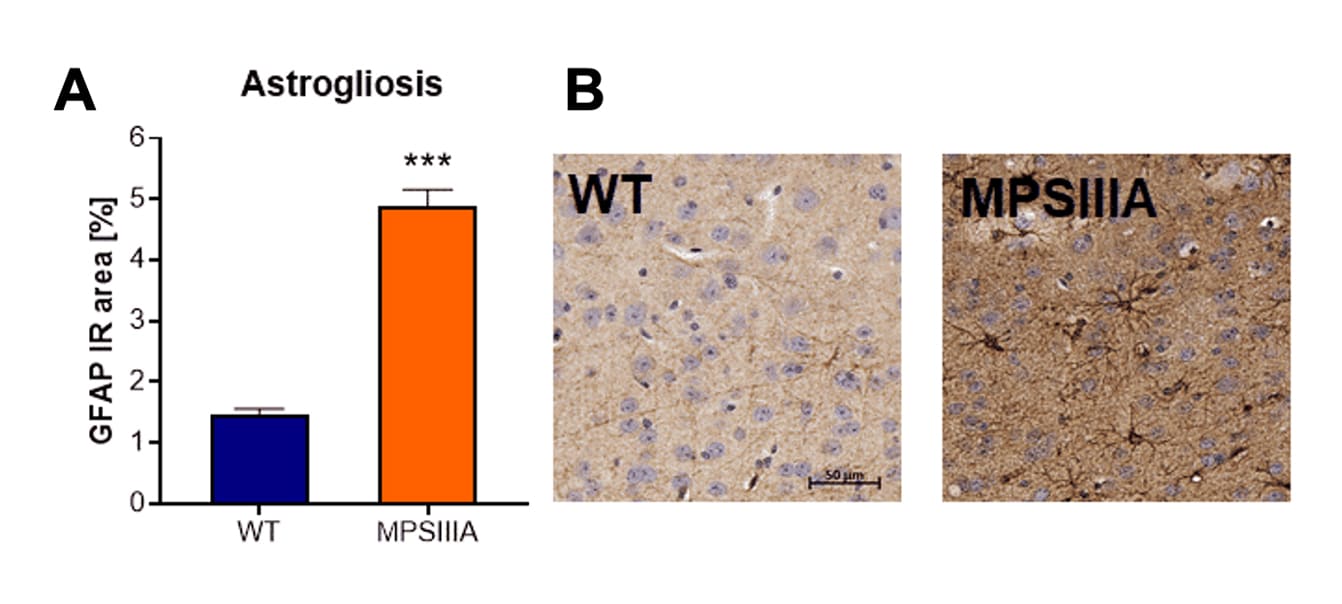
Figure 2: Histological analysis of MPSIIIA mice compared to wild type (WT) littermates for cortical neuroinflammation. A: Immunoreactive area (IR) in percent of GFAP labeling. Mean + SEM; unpaired t-test. ***p<0.001. B: Representative images of GFAP labeling in the cortex of MPSIIIA and WT animals at the age of 30 weeks.
Histological evaluation of lysosomal and endosomal membranes by LIMP2 labeling showed a highly increased LIMP2-positive immunoreactive area in the cortex of MPSIIIA mice compared to wild type mice (Fig.3A and B).
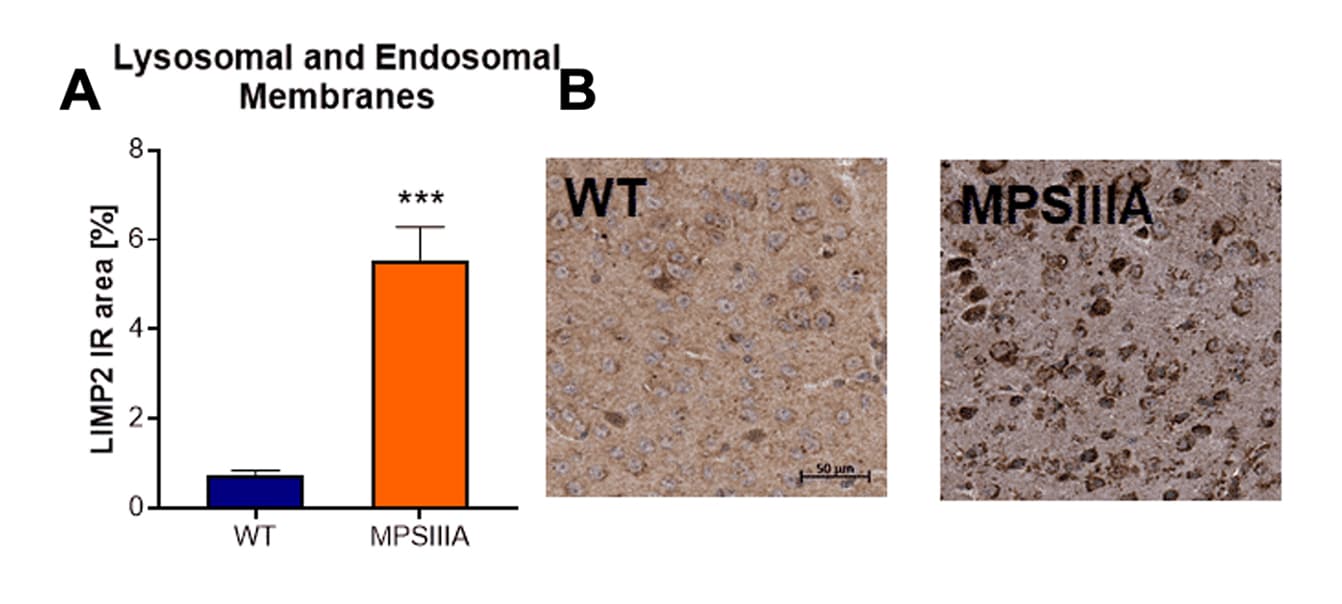
Figure 3: Histological analysis of MPSIIIA mice compared to WT littermates for cortical lysosomal/endosomal membrane alterations. A: Immunoreactive area (IR) in percent of LIMP2 labeling. Mean + SEM; unpaired t-test. ***p<0.001. B: Representative images of LIMP2 labeling in the cortex of MPSIIIA and WT animals at the age of 30 weeks.
QPS Neuropharmacology offers a custom-tailored study design for MPSIIIA mice, and we are flexible to accommodate to your special interest. We are also happy to advise you and propose study designs. MPSIIIA mice show a relevant Mucopolysaccharidosis IIIA phenotype Furthermore, wild type littermates are available as control animals needed for proper study design.
We are happy to evaluate the efficacy of your compound in the MPSIIIA mouse model!
The most common readouts are:
You might be also interested in these related topics:
- 4L/PS-NA Gaucher Disease Mouse Model
- NPC1-/- Niemann-Pick C1 Mouse Model
- Pompe 6neo Mouse Model
- In Vitro Models
