The pathological aggregation of amyloid-β peptides (Aβ) is one of the major causes for the progressive cognitive decline in AD patients. The development of compounds interfering with Aβ aggregation and thus able to rescue neurodegeneration is indispensable. For this purpose, fast and reliable assays are needed that are capable of showing direct effects of compounds on Aβ oligomer formation.
QPS Neuropharmacology exclusively provides a fast and reproducible screening assay, Amorfix Aggregated Aβ Assay (A4), that is able to directly visualize beneficial effects of your developmental compounds on the formation of Aβ aggregates in vitro. Kinetics of Aβ aggregation can also be monitored over time by using Thioflavin T dye (ThT). The assay is based on the property of ThT dye in which fluorescence (Ex/Em=440/484 nm) is increased when bound to aggregated Aβ peptides.
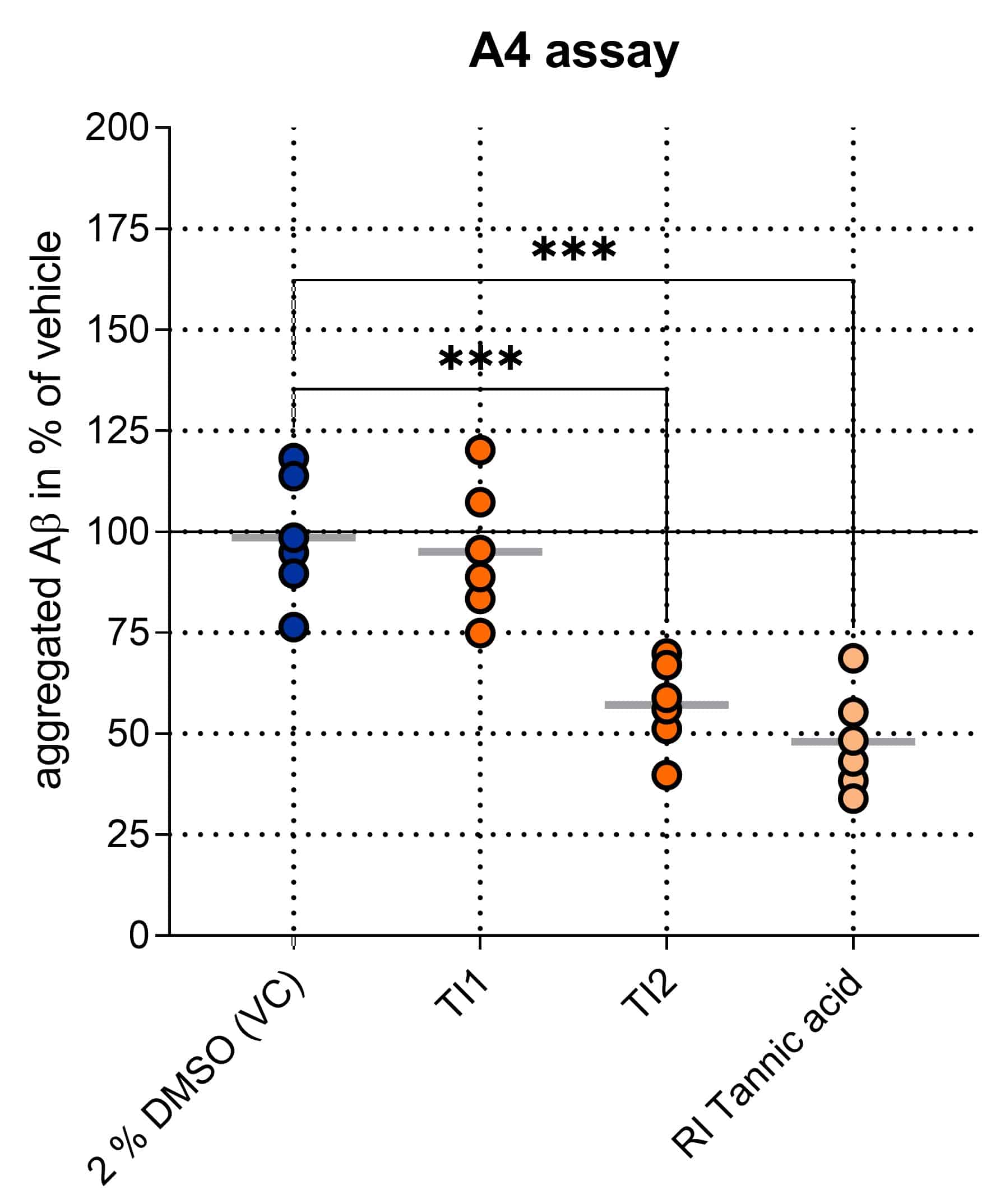
Figure 1: Determination of Aβ oligomers in vitro by A4 Assay. Compounds were co-aggregated with Aβ1-42 for 48 h in vitro. Aggregated Aβ was separated from monomers through affinity interaction. After disaggregation, the originally aggregated Aβ was detected using an immunosorbent assay. Aβ levels were evaluated as % aggregated Aβ of vehicle control (VC). Mean ± SEM; One-way ANOVA with Bonferroni‘s post hoc test; ***p<0.001.
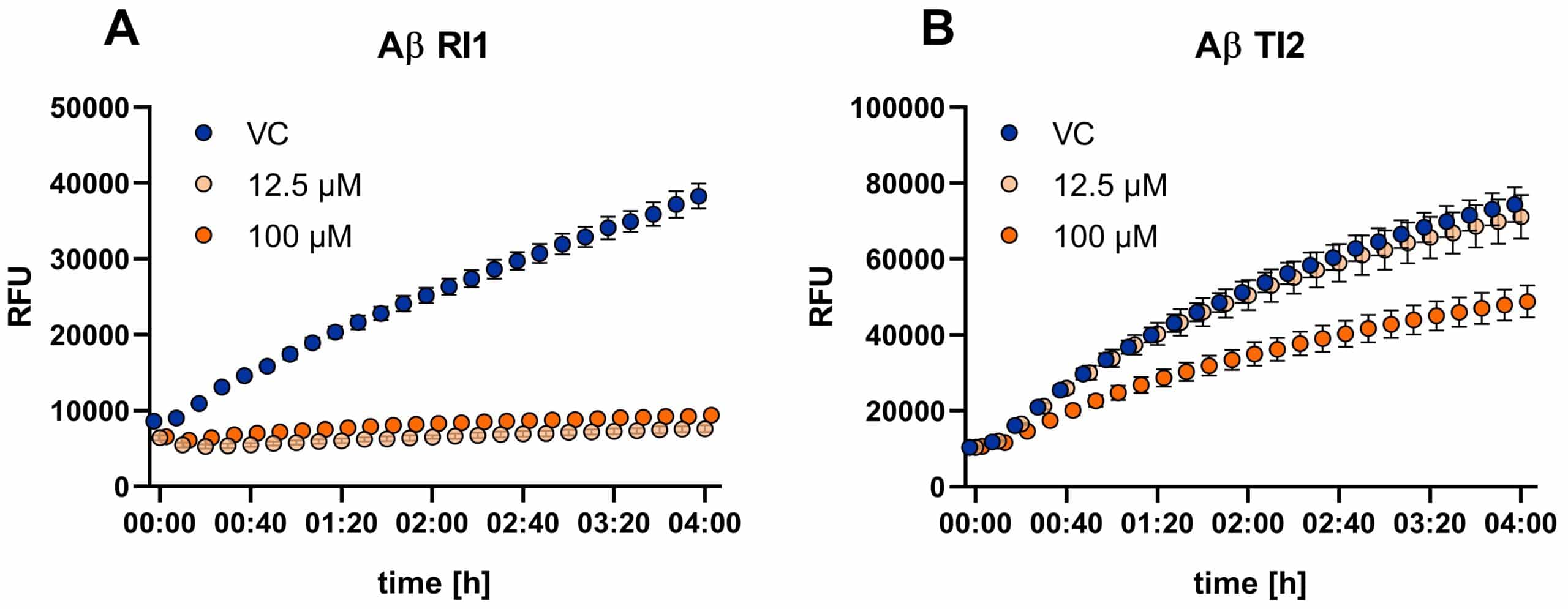
Figure 2: Determination of Aβ oligomers in vitro by ThT Assay. Compounds were co-aggregated with Aβ1-42 and ThT and kinetics of Aβ aggregation are monitored over time as increase in green fluorescence. Data are presented as RFU (relative fluorescence unit). The reference item (A; RI, tannic acid) as well as the test item (B) at 100 µM concentration were found to reduce Aβ aggregation. Mean ± SEM.
